Clinical prognostic models help clinicians tailor treatments for alterations in the brain-body interface to maximize chances of survival and recovery after aneurysmal subarachnoid hemorrhage. This article uses regression analysis, classification and regression tree analysis, as well as machine learning technique of artificial neural networks to create a prognostic decision making tool for cerebrovascular disorders.
Clinical outcome prediction model was created using the 3551-patient Tirilazad database to investigate clinical factors that influence outcome in patients with ruptured brain aneurysms (Figure 1). Dependent variable used for statistical analysis is the dichotomized Glasgow Outcome Score (GOS) at three months post aneurysmal subarachnoid hemorrhage. Good outcome represents functional independence (GOS 5 or 4). Poor outcome represents functional dependence (GOS 3), persistent vegetative state (GOS 2) or death (GOS 1).
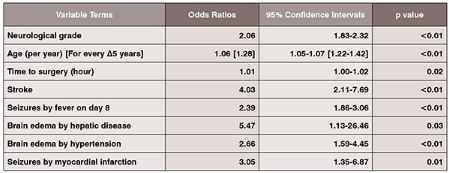
The main effects logistic regression model confirmed the significance of neurological grade, age, stroke, and time to surgery in outcome prognosis in aneurysmal SAH (Table 1).
This study also demonstrates that the odds of poor neurological outcome is increased by a factor of 4 in aneurysmal SAH patients who develop post-admission strokes (OR: 4.03, 95% CI: 2.11–7.69, p < 0.01). Cerebral infarction after aneurysmal SAH may occur early after aneurysmal rupture or in a delayed manner. Factors associated with the development of cerebral infarction include admission neurological status, treatment related complications, and occurrence of symptomatic vasospasm.
In addition, we make the observation that development of cerebral edema, in the context of history of hypertension and liver disease, has a significant impact on neurologic outcome deterioration in aneurysmal SAH. By itself, the development of cerebral edema may predispose the aneurysmal SAH patient to poor neurological outcome. Examination of systemic factors revealed that aneurysmal SAH patients with a history of hypertension and development of cerebral edema have 2.7 fold increased odds of poor neurologic outcome (OR: 2.66, 95% CI 1.59–4.45, p< 0.01). Patients with a history of hypertension are prone to defective cerebral autoregulation. When disrupted cerebral autoregulation is present after aneurysmal SAH, brain engorgement can occur as plasma proteins leak from capillaries with increased permeability. Extracellular vasogenic edema may follow as a result of increased hydrostatic pressures, with a predilection for posterior cerebral circulation territories.
This study also makes the observation that development of cerebral edema in aneurysmal SAH patients with a history of liver dysfunction markedly increases likelihood for poor outcome (OR: 5.47, 95% CI: 1.13–26.46, p = 0.03). Similar to patients with hypertensive history, patients with chronic liver disease have been shown to have altered cerebral autoregulation and cerebral blood flow with decreased cerebral blood flow in the anterior cingulum and increased blood flow in the basal ganglia and occipital lobes at baseline. In acute states of ruptured cerebral aneurysms, hese patients’ blood brain barriers become disrupted with marked increased cerebral blood flow secondary to luxury perfusion, thus, predisposing them to development of vasogenic edema. In addition, cytotoxic osmoregulatory mechanisms are involved whereby astrocytes swell secondary to the toxic effects of ammonia and glutamate. The end result is a vicious cycle of neuronal swelling and death, a marked increase in cerebral blood flow (cerebral hyperemia), and cerebral edema. It is important, therefore, to prevent the development of hepatic encephalopathy and monitor or cerebral edema in aneurysmal SAH patients who have chronic liver dysfunction.

Seizures increase mortality after cerebral aneurysmal rupture. In this study, we observed that seizures in the clinical setting of post–admission fever and background history of myocardial infarction significantly increase morbidity and mortality. The epileptic aneurysmal SAH patient who develops post–admission fever is predisposed to poor outcome (OR: 2.39, 95% CI: 1.86–3.06, p< 0.01). Fevers increase cerebral metabolic rate and can exacerbate the secondary injury. Early onset fevers can be secondary to dysfunction of temperature regulation centers in the hypothalamus whereas late onset fevers are more likely to be infectious, but can include fevers secondary to central damage, drugs, deep venous thrombosis andpulmonary embolism.
Exogenous and endogenous pyrogens increase the propensity for fevers and seizure development. In febrile states, inflammatory cytokines increase neuronal excitability via temperature sensitive ion channels leading to the increased likelihood of synchronized neuronal activity. Not only is it essential to monitor, prevent and treat both seizures and fevers themselves, it is also important to search for underlying etiologies, including infections, venous thrombosis and pulmonary embolism, drug-drug interactions and delayed strokes which may alter seizure thresholds in the febrile aneurysmal SAH patient.
Lastly, our regression analysis makes the observation that seizures in the setting of a history of myocardial infarction increase the odds of poor outcome by a factor of 3.05 (95% CI: 1.35–6.87, p = 0.01). Repetitive autonomic stimulation can occur in the actively seizing aneurysmal SAH patient in a lock-step phenomenon, which can trigger the development of cardiac ictal arrthymias. Continuous cardiac sympathetic discharges and cortical epileptiform activity can occur in a synchronized time-locked manner. These repetitive synchronized autonomic sympathetic discharges lead to cardiac ischemia and structural damage to the myocardium. In aneurysmal SAH, patients with pre-existing coronary artery disease and seizures, the propensity of cardiac ischemia is increased, along with potentially fatal multi-systemic complications, including development of neurogenic pulmonary edema and respiratory suppression associated with fatal cardiac tachyor brady-arrthymias, or cardiac asystole. Multi-system critical care cardiovascular and respiratory supports, therefore, are essential in these epileptic aneurysmal SAH patients in order to maximize their chances of survival.
Clinical prediction tools facilitate the process of prognostication and clinical decision making for both clinicians and patient families. In the 3551-patient Tirilazad database, unfavorable outcome (functional dependence, persistent vegetative state, and death) at three months after aneurysmal rupture was observed in 1061 (30%) of patients. Our current classification and regression tree makes use of the two most frequently retained clinical outcome, namely, neurological grade and age. It also demonstrates the significance of both post-admission stroke and fever in outcome prediction (Table 2).
In the present study, the occurrence of post-admission stroke increases the proportion of unfavorable neurologic outcome in aneurysmal SAH patients originally presenting with favorable admission neurological grades by 30%. Patients experiencing vasospasm are at an increased risk of post-admission strokes. In addition, several secondary injury cascade events may predispose these patients to post-admission strokes, including: (1) microthrombi formation, (2) cortical spreading depression, (3) microvascular constriction, (4) proliferation of pro-inflammatory cascade, (5) presence of blood–brain barrier disruption, and (6) inadequate collateral circulation.
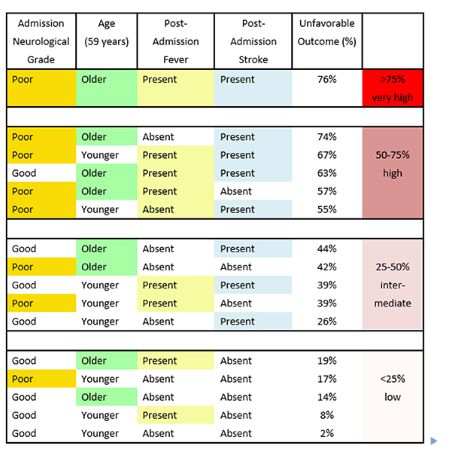
Fever is often a clinical indicator of neurological deterioration because it also triggers events in the secondary cascade of neurological injury. The various causes of post-admission late onset fevers, including nosocomial infections, central neurological injury, thromboembolic events, and drugdrug interactions, can lead to neurological complications, including increased intracranial pressures, cerebral edema, and post-admission strokes. Aggressive symptomatic control and rigorous search for underlying etiology are, therefore, warranted.
Clinical Outcome Prediction in Aneurysmal Subarachnoid Hemorrhage using Machine Learning Techniques
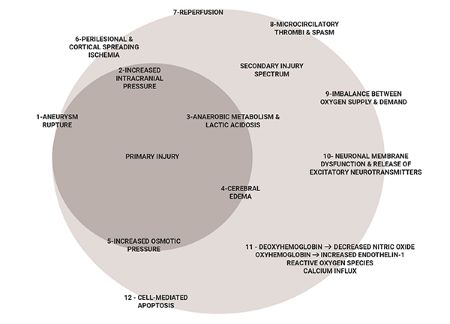
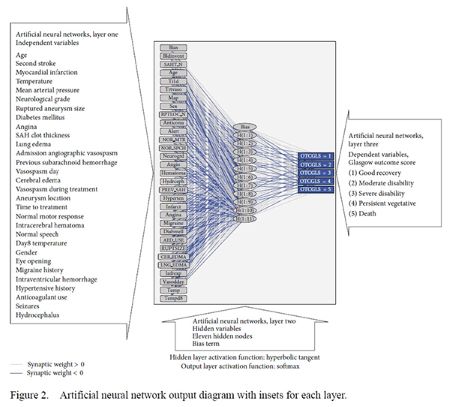
Exploratory analysis using artificial neural networks reveals the complexity of brain-body interactions in aneurysmal subarachnoid hemorrhage (Figure 2). In addition to the aforementioned variables, other variables, not directly measured, also influence clinical outcomes in aneurysmal subarachnoid hemorrhage patients. These include:
1. Disrupted cerebral autoregulation contributing to both ischemia and cerebral edema after subarachnoid hemorrhage.
2. Biochemical markers of brain injury predisposing to cortical spreading depression.
3. Cellular markers demonstrating physiologic dysfunction (such as mitochondrial dysfunction as reflected by the imbalance of oxygen supply and consumption).
4. Genetic factors affecting the outcome (such as inheritance of genetic markers predisposing to micro-thrombotic events in the cerebral microvasculature disrupting cerebral blood flow).
5. Multiple drug-drug interactions, especially in the elderly aneurysmal subarachnoid hemorrhage patient population with multiple pre-existing comorbidities, and
6. Multi-organ systemic dysfunctions, including interactions between the central nervous system and neuro-endocrinologic, metabolic homeostasis, hematologic, hepatic and splenic systems.
Conclusion
Using clinical prognostic models, the clinician can tailor individual specific treatment efforts to prevent and treat various alterations in the brain-body interface in order to maximize the chances of survival and recovery after aneurysmal subarachnoid hemorrhage.
Acknowledgement
Dr. Loch Macdonald is acknowledged for his expertise in this clinical area as well as the provision of a database.
References
1. Cook RJ, Lee KA, Lo BWY, Macdonald RL. Classical Regression and Predictive Modeling. World Neurosurg. 2022 May;161:251-264.
2. Lo BWY, Fukuda H. Pathophysiology of Brain-Body Interactions in Aneurysmal Subarachnoid Hemorrhage. New York: Nova Biomedical; 2018.
3. Lo BWY, Levine M, Thabane L, Farrohkyar F. The Brain-Body Interface in Aneurysmal Subarachnoid Hemorrhage – Outcome Prognostication and Creation of Decision Making Algorithm.; 2016. http://hdl.handle.net/11375/20698.
4. Lo BW, Fukuda H, Angle M, Teitelbaum J, Macdonald RL, Farrokhyar F, Thabane L, Levine MA. Clinical outcome prediction in aneurysmal subarachnoid hemorrhage - Alterations in brain-body interface. Surg Neurol Int. 2016 Aug 1;7(Suppl 18):S527-37.
5. Lo BW, Fukuda H, Angle M, Teitelbaum J, Macdonald RL, Farrokhyar F, Thabane L, Levine MA. Aneurysmal subarachnoid hemorrhage prognostic decision-making algorithm using classification and regression tree analysis. Surg Neurol Int. 2016 Jul 7;7:73.
6. Lo BW, Fukuda H, Nishimura Y, Macdonald RL, Farrokhyar F, Thabane L, Levine MA. Pathophysiologic mechanisms of brain-body associations in ruptured brain aneurysms: A systematic review. Surg Neurol Int. 2015 Aug 11;6:136.
7. Lo BW, Fukuda H, Nishimura Y, Farrokhyar F, Thabane L, Levine MA. Systematic review of clinical prediction tools and prognostic factors in aneurysmal subarachnoid hemorrhage. Surg Neurol Int. 2015 Aug 11;6:135.
8. Lo BWY, Fukuda H, Nishimura Y, Wan YY, Lo AWM. Brain-Body Interactions : Contemporary Outcome Prediction in Aneurysmal Subarachnoid Hemorrhage Using Bayesian Neural Networks and Fuzzy Logic. New York: Nova Biomedical; 2014.
9. Lo BW, Macdonald RL, Baker A, Levine MA. Clinical outcome prediction in aneurysmal subarachnoid hemorrhage using Bayesian neural networks with fuzzy logic inferences. Comput Math Methods Med. 2013;2013:904860. doi: 10.1155/2013/904860.