Mechanical circulatory support is an important adjunct to the management of patients with advanced heart failure. Technology advances in this area have improved overall survival. The challenge for clinicians is to translate the clinical evidence into selection of the most appropriate device that will provide benefit for an individual patient.
Mechanical Circulatory Support (MCS) is an important adjunct to the management of patients with severe heart failure. Because the number of donor hearts available for transplantation is limited, the use of MCS is growing as a valid alternative to save the lives of patients who are facing death.1
There is substantial evidence that MCS is able to revert the cascade of pathophysiologic events observed in patients with advanced heart failure. Although there are established protocols to assess ventricular
recovery post-MCS, there is still no parameter available that allows estimation of how long the improved cardiac function will persist.
Let us have a look at the current clinically available mechanical support devices, their indications for use and the specific advantages and disadvantages associated with each device.
Indications for support
MCS is a life-saving option for the patients who fail to improve or stabilise with intravenous inotropes or vasodilators, intra-aortic balloon pump support, and mechanical ventilation. Patients requiring mechanical support generally fall into four categories: those with 1. Cardiogenic shock resulting from Acute Myocardial Infarction (AMI); 2. Post-surgical myocardial dysfunction; 3. Acute cardiac failure from myocarditis and 4. Decompensated chronic heart failure.2
Patients who present a cardiogenic shock after an AMI are excellent candidates for either short- or long-term mechanical support because they have not developed the systemic organ dysfunction seen with chronic end-stage heart failure and have the potential for myocardial recovery.
For patients with the potential for recovery, temporary short-term support should be considered for a period of 5-7 days. Patients who fail to demonstrate myocardial recovery within seven days should be considered for conversion to a long-term device. In patients who are not eligible for transplant, device withdrawal should be considered if destination therapy with a long-term implantable device is not an option.
Patients with post-surgical shock can be divided into 1) Those with pre-existing ventricular dysfunction and therefore a low chance of recovery and 2) Those who had normal ventricular function before surgery and may recover with short-term support.
An Abiomed BVS 5000 may be the most appropriate choice for the patient with previously normal cardiac function while immediate use of an implantable left ventricular assist device (LVAD) may be the wisest choice in patients with pre-existing severe myocardial dysfunction.3,4
 Acute myocarditis is another common indication for cardiac mechanical support. Short-term support is indicated in patients with persistent hemodynamic instability despite maximal medical therapy. Failure to demonstrate adequate myocardial recovery should prompt evaluation for conversion to a long-term device.
Acute myocarditis is another common indication for cardiac mechanical support. Short-term support is indicated in patients with persistent hemodynamic instability despite maximal medical therapy. Failure to demonstrate adequate myocardial recovery should prompt evaluation for conversion to a long-term device.
Decompensation of chronic heart failure is the most common indication for long-term MCS.
Goals of mechanical circulatory support
The majority of experience with MCS has occurred in patients supported temporarily as a bridge to transplantation.
One important observation during the bridge-to-transplant experience was that some hearts recovered sufficient function to have the device removed. Given the shortage of donor organs, all patients undergoing MCS should be systematically evaluated for evidence of myocardial recovery. The bridge-to-recovery will be most successful in patients with post-surgical cardiac failure, acute myocarditis and AMI who have a high chance for cardiac improvement in accordance with the nature of their diseases.
The use of LVADs as an alternative to heart transplantation (destination therapy) has demonstrated significant survival benefits in these patients.5,6
Ventricular assist devices
There are several FDA-approved Ventricular Assist Devices (VAD), in addition to the intra-aortic balloon pump.7
Extracorporeal devices include the Abiomed BVS 5000 and Thoratec, which are both capable of biventricular assistance. Implantable devices designed for left ventricular support are the Novacor N1000PC, the HeartMate Pneumatic and the Vented Electric LVADs.
The next-generation devices consist of axial flow pumps with non-pulsatile flow, totally implantable LVADs. The HeartMate II LVA System (Thoratec), MicroMed DeBakey VAD System (MicroMed), Jarvis 2000 Heart (Jarvik Heart) and the VentrAssist LVA System (Ventracor) are the subject of ongoing clinical evaluation.
Extracorporeal Devices
Abiomed BVS 5000
The Abiomed BVS 5000 is an external, pulsatile, mechanical support system that can be used for univentricular or biventricular support. The advantages of this support system are its ease of use and availability. Thromboembolic, bleeding and infectious complications limit support periods generally to less than 14 days.
Thoratec
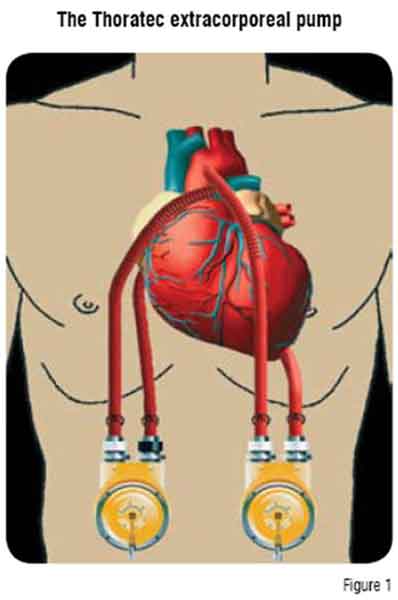
The Thoratec paracorporeal pump is a pneumatically driven, polyurethane sac designed for long-term use (Figure 1). The Thoratec VAD system is indicated as a bridge to transplantation and a bridge to recovery.
The pump is positioned on the external abdominal wall with cannulae tunnelled subcostally into the mediastinum. The Thoratec VAD provides uni- or bi-ventricular support.8 These cannulae are connected to an external pump (one for each ventricle), consisting of a rigid housing chamber containing a polyurethane blood sac. An external drive console sends pressurised air to the pump, compressing the blood sac and ejecting blood through mechanical valves.
The external position of the pump allows device exchange in cases of malfunction, thrombus or infection. Furthermore, this also enables use in patients who are poor candidates for implantable devices. Patients require systemic anticoagulation for the duration of the Thoratec VAD implantation.
Continuous flow pumps
Axial or rotatory blood pumps have been developed with the goal of intermediate-term as well as long-term ventricular assistance. These non-pulsatile-flow systems have shown some advantages in contrast to pulsatile systems: smaller size, higher efficiency, less infections, lower incidence of thromboembolic events and lower cost.9 Early clinical experience has shown that long-term non-pulsatile blood flow is well tolerated.
Intracorporeal devices
HeartMate
The HeartMate LVAD is implanted in a preperitoneal pocket, anterior to the posterior rectus sheath and just below the left costal margin (Figure 2). The inflow cannula is connected to the apex of the left ventricle and the outflow cannula is anastomosed to the ascending aorta. There are two types of HeartMate devices. The Implantable Pneumatic LVAD (IP-LVAD) is powered and controlled by an external pneumatic drive console that rests on a wheeled cart. The Vented Electric LVAD (VE-LVAD) contains an electric motor within the blood pump housing. It receives external power and control signals from an external microprocessor via a vented drive-line. Both systems have porcine valves and textured blood-contacting surfaces that become covered by a ‘pseudoneointimal’ layer. This results in a very low incidence of thromboembolic events and, therefore, patients do not require systemic anticoagulation.
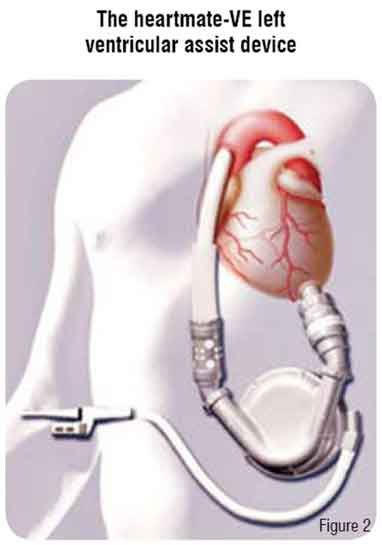 Insertion of the HeartMate is difficult in patients with a body surface area less than 1.5 m2 due to anatomical constraints. The major complications occur early and include haemorrhage and right heart failure. Infection remains a common complication (30-50 per cent) with prolonged use and is the biggest impediment to long-term success.
Insertion of the HeartMate is difficult in patients with a body surface area less than 1.5 m2 due to anatomical constraints. The major complications occur early and include haemorrhage and right heart failure. Infection remains a common complication (30-50 per cent) with prolonged use and is the biggest impediment to long-term success.
Novacor
The Novacor is an implantable, electric, dual pusher plate device designed for long-term cardiac support. The pump housing is constructed of a smooth polyurethane pump sac with gelatin-sealed inflow and outflow polyester grafts containing porcine bioprosthetic valves.
The Novacor shares many similarities with the HeartMate system including an external drive system with a portable power pack option. The device is implanted via sternotomy with an inflow conduit to the left ventricular apex and an outflow conduit to the ascending aorta. The pump itself is positioned in an abdominal subfascial plane or intraperitoneally with the tunnelled drive-line exiting the abdominal wall. A console or portable system regulates the pumping rate. The Novacor LVAD device requires systemic anticoagulation to prevent thromboembolism (risk 5-7 per cent). The incidence of primary device failure is very rare.10
CardioWest Total Artificial Heart (TAH)
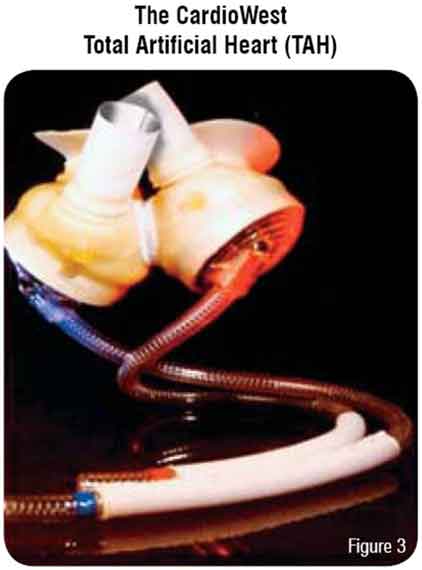
The CardioWest is currently the only total artificial heart approved for use in the US under an FDA investigational device exemption (Figure 3). This device is pneumatically driven and implanted in the orthotopic position. The pump consists of a rigid pump housing that contains dual spherical polyurethane chambers. The dual ventricular chambers are anastomosed to native atrial cuffs and the outflow conduit is anastomosed to the great vessels. Dual pneumatic drive-lines exit transcutaneously to a console control system which monitors pump pressures and performance. Antiplatelet and systemic anticoagulation are needed.11,12 This device is used as bridge-to-transplant in patients with biventricular failure.
 AbioCor TAH
AbioCor TAH
The AbioCor TAH is the first fully implantable replacement heart. It has been approved by the FDA as an investigational new device to be tested on selected patients.13,14 The AbioCor consists of an internal thoracic unit, an internal rechargeable battery, an internal miniaturized electronics package and an external battery pack. The thoracic unit is equipped with an internal motor that is able to move blood through the lungs and the rest of the body. The use of transcutaneous energy transmission eliminates the need for the patient to be immobilised permanently by tubes or wires connected to an external power source thus possibly reducing risk of infections.
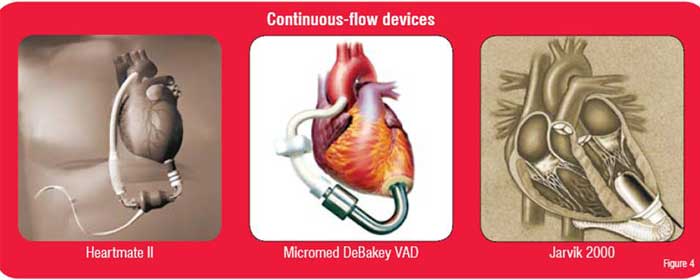
Devices in clinical trials include the Heartmate II, the Micromed DeBakey VAD, and the Jarvik 2000 (Figure 4). The DeBakey pump has already been successfully implanted in a small number of patients in Europe.15,16 The Heartmate II and the Jarvik 2000 have also been successfully implanted in humans.17,18 Unfortunately, there are few options or backup mechanisms other than replacement. Additionally, since these devices do not have valves, if a malfunction occurs the patient may develop the equivalent of wide-open aortic insufficiency.
Short-term support provided by centrifugal pumps has been shown to be a safe and simple cardiac support system with an overall wean rate of 50 to 60 per cent and a survival to discharge rate of 25 to 40 per cent.19,20,21 The use of short-term devices in selected high-risk patients as a bridge to long-term devices has shown survival rates no significantly different from the survival rate after long-term support alone.22
Successful transplantation is accomplished in 60 to 65 per cent of patients who received a long-term device. Between 28 to 38 per cent of all supported patients are discharged from hospital and managed as outpatients. Patients with LVAD have a higher survival to transplantation rate than the non-LVAD patients.23
CardioWest TAH provided a survival to transplantation rate of 75 per cent and a survival rate post-transplant >80 per cent There are limited published data regarding axial flow pumps, AbioCor TAH, and other LVADs; however, early results have shown safety, efficacy and reliability.
Device Selection
Device selection depends not only on specific patient characteristics and the etiology of the patient’s heart failure, but also on device characteristics, device availability and the experience of the surgical team.24
Patients in profound post-surgical cardiogenic shock require support to avoid permanent end-organ dysfunction and increase their chances of survival. The preferred devices are the Abiomed BVS 5000 and Thoratec device. These devices may provide full biventricular support re-establishing near normal haemodynamics while awaiting myocardial recovery. If prolonged support is expected, conversion to a longer-term device such as an implantable LVAD or TAH should be considered. The Thoratec device has the advantage of providing long-term, extracorporeal support.
Device selection for long-term support is much more complicated and often is subjective and based on the surgeons experience and bias. For smaller patients (BSA <1.5m2), the Thoratec device and eventually a continuous flow pump are the only options. For the larger patient, all devices are potential options. Most frequently an implantable LVAD is used but the CardioWest is useful for severe biventricular failure.
Summary
Mechanical circulatory systems have been shown to be an effective short-term therapy as a bridge to transplantation and as permanent cardiac support. The technological and human resources required to implement a mechanical assist device programme represent major limitations. Unfortunately, this technology is currently used only in dedicated centres.
The next generation of mechanical assist devices will provide hope for the burgeoning number of patients with end-stage heart failure, regardless of their eligibility for transplantation.
AUTHOR BIO
Diego Delgado is an Assistant Professor in the Division of Cardiology and Transplantation at the Toronto General Hospital, Canada. His interests are immunologic aspects of heart failure and transplantation. He is the Past-Chair of the Canadian Cardiac Transplant Group. He is the Vice-President of the Interamerican Society of Cardiology.
References
1. Delgado DH, Rao V, Ross H, Verma S, Smedira N. Mechanical circulatory assistance: state of art. Circulation. 2002 Oct 15;106(16):2046-50
2. Delgado MS, Bernabeo G and Delgado DH. Progress in Mechanical Circulatory Support. Rev Esp Cardiol 2008 Jun;61 Suppl 2:25-32
3. Smedira N, Blackstone EH. Postcardiotomy mechanical support: risk factors and outcomes. Ann Thorac Surg 2001; 71: S60-66
4. El-Banayosy A, Korfer R, Morshuis M et al: Bridging to Cardiac Transplantation with the Thoratec Ventricular Assist Device. Thorac Cardiovasc Surg 1999; 47 (suppl 2): 307-310
5. Rose E A, Gelijns A C, Moskowitz A J, et al. Long-term left ventricular assistance for end-stage heart failure. N Engl J Med 2001; 345:1435-1443
6. Rogers JG, Butler J, Lansman SL, et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol 2007 Aug 21; 50(8): 748-751
7. Gomez Bueno M, Segovia Cubero J, Alonso-Pulpon L. Asistencia mecanica circulatroia y transplante cardiaco. Indicaciones y situacion en Espana. Rev Esp Cardiol Supl 2006; 6: 82F-94F
8. Rao V, Oz MC, Edwards NM et al. A new off-pump technique for Thoratec right ventricular assist device insertion. Ann Thorac Surg 2001; 71: 1719-1720
9. Nose Y, Tsutsui T, Butler K et al: Rotary Pumps. New Developments and Future Perspectives. ASAIO 1998; 44(3): 234-237
10. Degenaris F, Portner PM, Robbisns RCc and Over PE. The Novacor left ventricular assist system: clinical experience from the Novacor registry. J Card Surg. 2001;16(4):267-71
11. Copeland JG, Smith Rg, Arabia FA, et al; CardioWest Total Artificial Heart Investigators. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004 Aug 26; 351(9): 859-67
12. Copeland JG, Smith Rg, Arabia FA, et al; CardioWest Total Artificial Heart Investigators. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004 Aug 26; 351(9): 859-67
13. Zareba KM. The artificial heart: past, present and future. Med Sci Monit 2002; 8(3): 72-77
14. Frazier OH, Dowling RD, Gray LA, Shah NA, Pool T and Gregoric I. The total artificial heart: where we stand. Cardiology 2004; 101:117-121
15. Wieselthaler GM, Schima H, Hiesmayr M, et al. First clinical experience with the DeBakey VAD continuous-axial-flow pump for bridge to transplantation. Circulation 2000; 101(4):356-359
16. Potapov EV, Loebe M, Nasseri BA, et al. Pulsatile flow in patients with a novel nonpulsatile implantable ventricular assist device. Circulation 2000; 102(19 Suppl 3):III183-III187
17. Miller L, Pagani F, Russell S, et al. for the HeartMateII Clinical Investigators. N Engl J Med 2007; 357: 885-896
18. Westaby S, Banning AP, Jarvik R, et al. First permanent implant of the Jarvik 2000 Heart. Lancet 2000; 356(9233):900-903
19. Neon GP, Lafuente JA and Irwin S. Acute and temporary ventricular support with BioMedicus centrifugal pump. Ann Thorac Surg 1999; 68: 650-654
20. Samuels LE, Holmes EC, Thomas MP, et al. Management of acute cardiac failure with mechanical assist: experience with the ABIOMED BVS 5000. Ann Thorac Surg 2002; S67-72
21. Dekkers RJ, FitzGerald DJ and Couper GS. Five-year clinical experience with ABIOMED BVS 5000 as a ventricular device for cardiac failure. Prefusion 2001; 16: 8-13
22. Pagani FD, AaronsonKD, Swaniker F et al. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann Thorac Surg 2001; 71:S77-81
23. Deng MC, Edwards LB, Hertz MI, et al. Mechanical Circulatory Support Device Database of the International Society for Heart and Lung Transplantation: Third Annual Report-2005. J Heart Lung Transplant 2005; 24: 1182-1187
24. Stevenson LW, Rose EA. Left ventricular assist devices. Bridges to transplantation, recovery, and destination for whom? Circulation 2003; 108: 3059-3063