As most inpatient revenue is dependent on Diagnosis Related Group payment, cost reduction is necessary to protect margins. This study examines the impact on inpatient reference test order spend when an efficient formulary, including reference test identification, relative cost, and turnaround time is embedded within an electronic order entry system.

Over the past few decades there has been enormous growth in healthcare expenditure in the United States. Furthermore, healthcare spending is projected to grow at an average rate of 5.8% per year and by 2024 will comprise 20.1% of gross domestic product. Due to the overwhelming costs attributable to healthcare delivery, payers ranging from Medicare and Medicaid to private health insurance companies are rapidly moving to pay for performance reimbursement strategies such as the Medicare Shared Savings Plan, Medicare Access & CHIP Reauthorization Act (MACRA), and other value-based payment models. Given these challenges, it is essential for healthcare systems to reduce unnecessary expenditures and maximize quality and value. Within this context, effective laboratory utilization is critical as laboratory testing is the single highest-volume medical activity. An estimated 13 billion tests are performed in the United States each year. In addition, approximately 70% of downstream medical decisions are based on laboratory results.
Laboratory testing is not typically associated with adverse events, but significant patient harm can occur if errors are made. The three most common causes of patient harm are ordering the wrong test, failure to retrieve test results, and misinterpreting a test result. Studies have also shown that 10-30% of laboratory tests are unnecessary or inappropriate and 5% of genetic tests are frank medical errors. For these reasons, there is a great deal of interest in developing techniques and strategies to ensure appropriate laboratory utilization.
Several articles describe examples of successful laboratory utilization strategies, including the creation of multidisciplinary formulary committees and implementation of electronic laboratory utilization management systems. The move toward creation of laboratory formularies is logical given exponential growth in diagnostic test development. By one estimate approximately 8-10 new genetic tests enter the healthcare market daily. With the expanding list of available tests, laboratory formularies provide guidance in appropriate test selection ensuring quality and value.
While creating a laboratory formulary via a multidisciplinary utilization committee and integrating utilization management into an electronic order entry system serves as a strong basis for laboratory utilization efforts, little has been published on the impact when information such as identification of reference tests (versus testing performed in house), approximate cost, and estimated turn-around time (TAT) are provided to clinicians within a provider order entry (POE) system.
This paper examines the financial impact of a laboratory formulary created at a regional tertiary hospital with 400-500 beds. The test formulary was embedded as a test menu – including identification of reference tests, approximate cost, and TAT estimates – in the inpatient physician order entry system. The focus of this paper is to examine the impact on test ordering patterns when identification of reference tests, approximate cost and TAT is embedded into the test names within a formulary in an inpatient POE system.
The hospital in this study is a non-profit, 400-500 bed, level 1 trauma center on the east coast of the United States, serving multiple surrounding rural counties. In addition, this hospital serves as a teaching hospital affiliated with a nearby university. During the one-year timeframe of this study, the hospital had approximately 24,600 inpatient discharges, approximately 265,000 outpatient visits, and the laboratory performed approximately 1,430,000 billable tests (combined in house and reference tests).
Prior to this study, the Medical Executive Committee of the study facility established a multidisciplinary Laboratory Utilization Committee chaired by a Pathologist with ad hoc members including department chairs of internal medicine and various subspecialties (oncology, infectious diseases, pediatrics, and gastroenterology). Key activities included consolidation of reference testing from multiple reference labs to a primary reference laboratory and reduction of redundant in-house tests.
In several instances, it was determined that the recent electronic medical record implementation had contributed to poor test utilization. For example, some tests were routed to the incorrect performing reference laboratory due to mismatched test codes, test naming conventions were non-standardized leading to ordering confusion, and to rapidly deploy order sets within the new order entry system not all order sets were reviewed by the laboratory to ensure appropriateness. Physicians were also encouraged to save preferential orders within the order entry system as “Physician Favorites” or “Preference Items” without secondary review.
Within the first-year, significant cost savings were achieved by laboratory utilization efforts – primarily through consolidation of reference testing to a single primary reference lab where possible, addressing the need for laboratory order-set review, and monitoring of physician favorites. Over this period of initial improvement, the actual volume of reference testing had not been reduced and volumes were still higher than prior to the transition to the order entry system. For these reasons, the committee decided to next attempt the creation of an inpatient reference test formulary.
When originally implemented, the inpatient order entry system laboratory menu was populated with 847 reference tests. Many were built as orderable for no other reason as they had been previously built in the laboratory information system. As a starting point for the draft formulary, the volume of reference testing over the preceding 12-month period was reviewed and reference tests with fewer than four tests ordered were excluded from the menu (approximately 400 unique tests). Select problematic tests ordered more than four times per year (Folate Red Blood Cell, Methylenetetrahydrofolate Reductase, 1, 25-Dihydoxy Vitamin D, etc.) and other tests determined by the committee members as likely being non-contributory to inpatient care were also excluded. Upon completion, the revised test menu in the order entry system decreased from 847 reference tests to 176 tests. Of note, while 671 tests had been eliminated, clinicians were still able to order “non-formulary” tests by free texting a special lab request to the laboratory with a brief explanation justifying the request.
During the process of creating the formulary, members of the multidisciplinary committee voiced concern over the inability of ordering physicians to readily determine which tests represented reference tests versus in-house tests, lack of knowledge regarding cost, and absence of TAT information. By adding TAT data it was proposed that inpatient test ordering decisions might be impacted by whether results would be available during a patient’s hospital admission versus post-discharge. While the physicians voiced interest in knowing this information, there was also great concern about the possible impact of including additional clinical decision support in the order entry system that may contribute to “pop-up” fatigue.
Based on these recommendations, the utilization committee elected to embed reference test identification, relative cost, and approximate TAT directly into the test name within the order entry system for all reference tests on the formulary. For example, “Homocysteine” became “Homocysteine (REF, $$, 3d)” wherein “REF” identified the test as a reference test, each “$” sign represented $50 cost increments based on institutional costing tool data, and “3d” represented an approximate three-day TAT.
Much discussion centered on the appropriate format of cost data within the test menu. Initial consideration was given to providing exact reference laboratory cost or patient charge but maintaining accurate pricing presented challenges given fluctuations in test cost over time. Also, secondary to wide variation in payer reimbursement (Medicare vs private insurance vs self-pay) determining potential out of pocket expense for patients was not feasible. Instead, by using “$” signs in $50 increments based on data from the institutional costing tool, a practical sense of relative cost scale was imparted rather than contending with possible pitfalls associated with providing the precise reimbursement or cost.
In summary, the formulary consisting of 176 reference tests (instead of 847) with embedded reference test identification and TAT became the list of orderable reference tests in the inpatient order entry system.
Reference test invoices from the primary reference laboratory were reviewed for the 12-month period prior to the revised formulary incorporation within the order entry system as were invoices from the 12-month period following implementation. Specifically, invoices were reviewed by hospital financial analysts to identify inpatient test cost, specifically excluding outpatient cost (not impacted by the changes made within the inpatient order entry system).
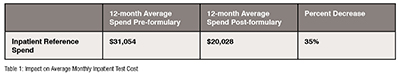
As demonstrated in Table 1, during the 12 months following the implementation of the lab formulary with reference test identification, relative cost, and TAT on all 176 reference tests, average inpatient reference costs per month decreased by 35% as compared to the average monthly cost calculated from the 12 months prior to implementation. This represents an average monthly savings of $11,026 with a projected yearly cost saving of $132,309.
While implementation of the inpatient reference test formulary reduced the average monthly inpatient reference spend by 35% during the study timeframe, average monthly inpatient admissions decreased by 5.1% and the case mix index (CMI) increased from 1.42 to 1.47 (3.5% increase). The precise impact of the small decline in admissions on inpatient reference test ordering is uncertain in the face of slightly increasing CMI, but the net result is unlikely to fully account for the overall 35% reduction in average monthly inpatient reference spend.
During the study timeframe, no other educational activities were employed to address misordered reference tests and the number of active medical staff at the study hospital remained relatively static. Therefore, the cost reduction does not appear related to a decrease in medical staff or possible turnover of high-volume ordering physicians.
In summary, the 35% decrease in monthly average reference lab cost appears to be the result of the formulary based upon removing tests ordered less than four times per year, elimination of tests not likely to contribute to inpatient care, and embedding identification of reference test, relative cost, and TAT in the order entry system.
No formal process was employed to assess medical staff response to implementation of the formulary but inquiries at various medical staff functions and committees were unanimously positive. Members of the medical staff voiced greater satisfaction due to streamlining of previously lengthy menus presenting numerous similar and generally unneeded tests. Staff comments also centered on appreciation for the identification of reference tests as most had little to no awareness of tests performed in house versus reference testing. Several providers voiced specific examples where test selection for specific patients was guided by comparing TAT to identify optimal inpatient diagnostic strategy. One factor that may have contributed to the overall level of physician support was the creation of the formulary based on the input of various members of the medical staff on the utilization committee and inclusion of their suggestions. In this regard, the formulary was more an example of physician engagement than strictly a cost-cutting tool.
The results demonstrate the benefit of creating an efficient inpatient reference test formulary that includes reference test identification, relative cost, and TAT data. Developing a laboratory formulary with an emphasis on removing infrequently ordered tests, deleting commonly misordered tests, and embedding reference test identification, cost, and TAT, decreases inpatient reference test spend. With more efficient test ordering, the downstream impact on medical decisions, while difficult to measure directly, likely generates exponential savings beyond that achieved for the laboratory budget alone. As healthcare systems face the challenge of transitioning to value-based payment models, optimal laboratory utilization strategies are critical to delivering high quality care while containing the growing costs attributable to healthcare in the United States.
References
1. National Health Expenditure Data Projected. Centers for Medicare & Medicaid Services. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/nationalhealthaccountsprojected.html.
2. CLIA Program and HIPAA Privacy Rule; Patients' Access to Test Reports. Federal Register. https://www.federalregister.gov/documents/2014/02/06/2014-02280/clia-program-and-hipaa-privacy-rule-patients-access-to-test-reports. Published February 6, 2014.
3. Green SF. The cost of poor blood specimen quality and errors in preanalytical processes. Clinical Biochemistry. 2013;46(13-14):1175-1179. doi:10.1016/j.clinbiochem.2013.06.001.
4. Plebani M. Exploring the iceberg of errors in laboratory medicine. Clinica Chimica Acta. 2009;404(1):16-23. doi:10.1016/j.cca.2009.03.022.
5. Gandhi TK, Kachalia A, Thomas EJ, Puopolo AL, Yoon C, Brennan TA, Studdert DM. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006 Oct 3;145(7):488-96. doi: 10.7326/0003-4819-145-7-200610030-00006. PMID: 17015866.
6. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013 Nov 15;8(11):e78962. doi: 10.1371/journal.pone.0078962. PMID: 24260139; PMCID: PMC3829815.
7. Patrick C. Mathias, MD, PhD and others, Preventing Genetic Testing Order Errors With a Laboratory Utilization Management Program, American Journal of Clinical Pathology, Volume 146, Issue 2, August 2016, Pages 221–226, https://doi.org/10.1093/ajcp/aqw105
8. Warren JS. Laboratory Test Utilization Program: Structure and Impact in a Large Academic Medical Center. American Journal of Clinical Pathology. 2013;139(3):289-297. doi:10.1309/ajcp4g6uauxcftqf.
9. Konger RL, Ndekwe P, Jones G, et al. Reduction in Unnecessary Clinical Laboratory Testing Through Utilization Management at a US Government Veterans Affairs Hospital. American Journal of Clinical Pathology. 2016;145(3):355-364. doi:10.1093/ajcp/aqv092.
10. Ray T. FDA to Finalize LDT Guidance Amid Uncertainty on Number of Genetic Tests Impacted. GenomeWeb. https://www.genomeweb.com/molecular-diagnostics/fda-finalize-ldt-guidance-amid-uncertainty-number-genetic-tests-impacted.
11. The Future of Lab Utilization Management. The Future of Lab Utilization Management - AACC.org. https://www.aacc.org/publications/cln/articles/2012/january/lab-utilization.